Thermal conductivity covers an essential segment of modern applied material science. It helps design heat sinks for electronics, optimize engine components, and develop energy-efficient building materials.
Aluminum has been one of the most widely featured materials across countless sectors. It’s well known for many great virtues. Additionally, the post-transition metal harnesses excellent thermal conductivity.
The article digs deep into aluminum thermal conductivity in understandable details. You’ll learn more than just values and units, as it explores how characteristics and alloy variations affect heat transfer.
What is the Thermal Conductivity of Aluminum?
As you should guess, it’s a fundamental property to quantify a material’s ability to conduct/transfer heat. Simply put, thermal conductivity measures how efficiently heat energy moves through a substance. Higher thermal conductivity means faster heat transfer.
The standard unit of thermal conductivity is watts per meter-kelvin (W/m·K). It expresses the amount of heat (watts) transferred through a one-meter thick material for each temperature difference (kelvin).
Importance of Thermal Conductivity for Aluminum
Ensure stable operation of equipment: In electronics (e.g., CPU, GPU), prevent overheating through heat dissipation; in automotive (e.g., engine, radiator), control thermal load and avoid breakdowns.
Improve energy efficiency: Reducing over-reliance on cooling/heating systems (e.g., building temperature control, electronic cooling) can reduce energy consumption of electronic devices by up to 30%.
Extended life and safety: Uniform heat dissipation reduces component thermal, extending the life of equipment/components and reducing the risk of overheating.
Aluminum Thermal Conductivity
Pure aluminum holds excellent thermal conductivity. It measures 237 W/m·K at room temperature (20°C). Such a high value stems from the metal’s free electron mobility. It allows rapid heat transfer through the atoms.
Comparison with Other Metals
- Silver (~428 W/m·K): Highest among metals. Expensive and rarely used for thermal purposes.
- Copper (~401 W/m·K): Superior to aluminum; widely used in heat exchangers and electronics.
- Aluminum (~237 W/m·K): Lightweight, cost-effective, and sufficiently conductive.
- Gold (~318 W/m·K): High conductivity, but impractical due to cost.
- Steel (~50 W/m·K): Much lower; used where strength matters more than heat transfer.
Copper surely snatches the victory in terms of thermal conduction value/quantity. However, aluminum offers a perfect balance of thermal performance, weight, and affordability.
Aluminum Conductivity Thermal Of Different Grades
Pure aluminum has almost no practical uses in industrial applications. Instead, it collaborates with elements like silicon (Si), copper (Cu), magnesium (Mg), and zinc (Zn).
They obtain a precise balance of desired mechanical properties for intended uses. However, these alloying elements disrupt the uniform atomic lattice. And it reduces the metal’s ability to conduct heat.
●Pure Aluminum (~237 W/m·K): Highest conductivity; used in heat sinks and electronics.
●6061-T6 Alloy (~166 W/m·K): Contains Mg and Si; common in structural applications.
●7075-T6 Alloy (~130 – 150 W/m·K): High strength, lower conductivity due to Zn and Cu content.
●Silumin or Al-Si alloy (~164 W/m·K): Si improves castability but reduces conductivity.
●Aluminum Bronze or Al-Cu (~70 W/m·K): Cu improves strength but weakens thermal action.
| Alloying Element | Effect on Thermal Conductivity | Aluminum Alloy Example | Alloy Conductivity (W/m·K) |
| Copper (Cu) | Massive reduction | 2024, 7075 | ~120 – 150 |
| Magnesium (Mg) | Moderate reduction | 5052, A356 | ~130 – 160 |
| Silicon (Si) | Depends on phase; though Si can help | 319, 380 | ~96 – 109 |
| Zinc (Zn) | Moderate reduction | 7075 | ~130 |
Some applications require high thermal conductivity. Choose alloys with minimal alloying content or those designed for thermal management. For example, get Al-Si alloys with controlled Si levels.
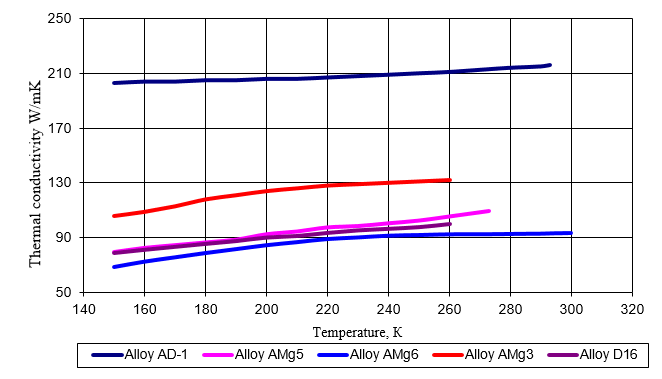
Thermal Conductivity Of Aluminum At Different Temperatures
Aluminum thermal conductivity can vary with temperature to some extent. Temperature increments push conductivity slightly below the standard due to enhanced atomic vibrations.
- At 20°C, ~237 W/m·K
- At 100°C, ~230 W/m·K
- At 300°C, ~232 W/m·K
- At 500°C, ~220 W/m·K
Such behavior is relatively common in metals with intense electron scattering at higher temperatures. However, aluminum maintains relatively stable conductivity across a broad range. That’s why it’s a reliable choice for fluctuating thermal environments.
Other Factors Affecting Thermal Conductivity Aluminum
1. Microstructure: Aluminum alloys may contain microscopic regions with distinctive compositions and structures. They can effectively enhance or hinder thermal conductivity.
2. Processing Techniques: Manufacturing methods (casting, heat treatment, and additive manufacturing) can alter aluminum’s thermal behavior. Heat treatment can precipitate alloying elements to improve conductivity.
3. Impurities: Even trace impurities can reduce conductivity by 10% – 30% based on concentration and distribution. Commercial-grade Al (99.5% purity) has lower conductivity than ultra-pure Al (99.99%).
4. Surface Treatments: Surface modifications like anodization can influence heat transfer. Anodized aluminum has a porous oxide layer. It slightly reduces thermal conductivity against increased emissivity.
5. Porosity: Solid aluminum sheet features a 100% relative conductivity. Meanwhile, open-cell foam can deliver only 10% – 30% of the value. Thin fins or sheets can cover almost 70% – 90% of the conductivity.
Applications of Thermal Conductivity Aluminum
1.Heat Exchangers and Cooling Systems
Aluminum is a top choice for heat exchangers, radiators, and cooling modules. It’s capable of rapidly transferring heat while maintaining structural integrity.
2.Electronics and Electrical Applications
As mentioned, aluminum plays a critical role in thermal management for electronic devices. They imply reliability along with steadfast performance.
3.Automotive and Aerospace Industries
Al’s thermal conductivity supports engine cooling, battery management, and structural heat dissipation. It’s particularly important for high-demand environments.
4.Packaging and Cooking Equipment
Aluminum’s ability to evenly distribute heat makes it ideal for cookware and food packaging.
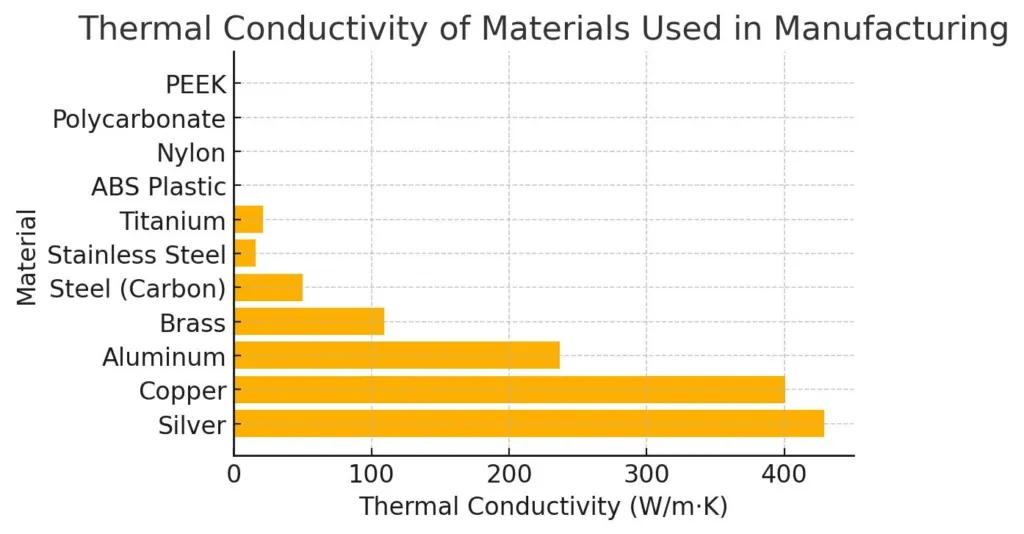
Materials with Better Thermal Conductivity (Alternative Choices)
Some applications call for maximum heat transfer. You may have to think of alternatives in those cases.
- Diamond (2000 – 2200 W/m·K): Highest known; used in high-end electronics and lasers.
- Silver (~429 W/m·K): Best among metals; expensive and rarely used in bulk.
- Copper (~398 W/m·K): Common in electronics; superior conductivity but heavier than aluminum.
- Gold (~315 W/m·K): Excellent but costly; used in aerospace and precision electronics.
- Aluminum Nitride (~310 W/m·K): High conductivity with electrical insulation for semiconductors.
- Graphite (~168 W/m·K): Lightweight and flexible; used in thermal interface materials.
Copper or aluminum nitride gets preference over aluminum for compact electronics. Their superior thermal performance and reliability under heat stress are sufficient.
Conclusion
Stable thermal conductivity is one of the foremost properties of aluminum for practical applications. A precise balance of different mechanical characteristics is what makes Al perfect. The metal’s efficient and reliable heat management continues to find new uses across many industries.
Get the Best Aluminum Parts for Your Projects from HRC
HRC Metal has been leading the CNC machining industry for 17+ years. We provide easy, one-stop, and committed solutions based on industry standards and cutting-edge technology. Whatever aluminum you need for your projects, contact us to know how we can meet your satisfaction.
Frequently Asked Questions (FAQs)
- Is aluminum a good heat conductor?
Yes. Al is considered an excellent heat conductor, second only to metals like Cu and Ag. It features a high thermal conductivity (~237 W/m·K). Aluminum also reflects infrared radiation to manage radiant heat.
- Why does Al have lower thermal conductivity than Cu?
Aluminum has fewer free electrons than copper, limiting its ability to transfer heat. Alloying with elements like Cu, Mg, Zn, or Si disrupts the lattice structure to reduce conductivity.
- How does surface treatment affect thermal conductivity?
Surface treatments like anodization or conversion coatings (Alodine, Iridite) can reduce aluminum’s thermal conductivity. These coatings form an oxide layer (Al₂O₃) with much lower conductivity (~30 W/m·K).
- How does aluminum’s crystal structure affect conductivity?
Aluminum has a face-centered cubic (FCC) crystal structure. Such a structure enables relatively higher thermal conductivity. The FCC arrangement also initiates uniform heat flow in all directions.
- Can processing techs enhance Al’s thermal conductivity?
Yes. Heat treatment, grain refinement, and controlled casting can improve thermal conductivity. Annealing reduces internal stresses and improves electron mobility. Directional solidification in casting can align grains to favor heat flow.
- How do high temperatures affect Al’s thermal conduction?
Aluminum’s thermal performance declines at elevated temperatures. Most Al alloys begin to lose strength above 150°C. 6061-T6 and 7075-T6 have maximum operating temperatures of 120°C – 150°C.

















